The Analysis of Gasoline
by RPW Scott
part of the Chrom Ed. Series
As with all small radius open tubular columns a split injection system must be used. In addition, the relatively wide boiling range of the gasoline will require a temperature program that will heat the column to 200oC or more and thus the stationary phase must be thermally stable. The components of the gasoline are present over a wide concentration range and thus, for accurate quantitative results, the linear dynamic range of the detector must also be large. These latter requisites mandates the use of an FID.
A separation of gasoline components is shown in figure 30. The stationary phase used was Petrocol which is the trade name for a special poly(dimethysiloxane) that is actually intra-column polymerized and thus bonded to the surface. As a result of the type of phase together with the nature of its bonding, it is very thermally stable. The alkane chains in the polymer gave strong dispersive properties to the stationary phase. The necessary high efficiency was obtained by using a 100 m column, 250 mm I.D. carrying a film of stationary phase 0.5 mm thick. The column was held at 35oC after in injection for 15 min and then programmed to 200oC at 2oC/min and finally held at 200oC for 5 min. The FID was held at 250 oC (50oC) above the maximum column temperature to ensure no condensation in the detector. The sample size was 0.1 ml which was split 100-1 onto the column and so the total charge on the column was about 1 mg. Helium was used as the carrier gas at a linear velocity of 20 cm/sec.
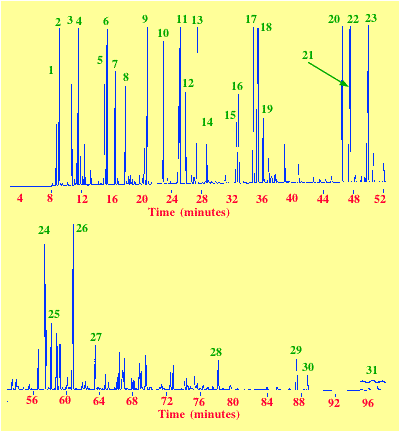
Courtesy of Supelco Inc.
| 1/ Isobutane | 2/ n-Butane | 3/ Isopentane |
| 4/ n-Pentane | 5/ 2,3-Dimethylbutane | 6/ 2-Methylpentane |
| 7/ 3-Methylpentane | 8/ n-Hexane | 9/ 2,4-Dimethylpentane |
| 10/ Benzene | 11/ 2-Methylhexane | 12/ 3-Methylhexane |
| 13/2,2,4Trimethylpentane | 14/ n-Heptane | 15/ 2,5-Dimethylhexane |
| 16/ 2,4-Dimethylhexane | 17/2,3,4Trimethylpentane | 18/ 2,3-Dimethylhexane |
| 19/ 2,3-Dimethylhexane | 20/ethylbenzene | 21/ m-Xylene |
| 22/ p-Xylene | 23/ o-Xylene | 24/ -Me-3-Ethylbenzene |
| 25/ 1,3,5TriMe-benzene | 26/ 1,2,4TriMe-benzene | 27/ 1,2,3TriMe-benzene |
| 28/ Naphthalene | 29/ 2-Methylnaphthalene | 30/ 1-Methylnaphthalene |
| 31/ Dimethylnaphthalene |
The value of the open tubular column is clearly demonstrated. This type of separation would be practically impossible using a packed column.
