The Electrical Conductivity Detectors
The electrical conductivity detector can only detect those substances that ionize and consequently, is frequently used in the analysis of inorganic acids, bases and salts. It has also found particular use in the detection of organic acids and bases that are frequently required in environmental studies and in biotechnology applications. The sensor is the simplest of all the detectors consisting of only two electrodes situated in a suitable flow cell.
An example of an electrical conductivity sensing cell is shown in figure 21. It consists of two electrodes situated in a suitable flow cell as depicted in the upper diagram. The electrodes are arranged to constitute one arm of a Wheatstone Bridge. When ions enter the detector cell, the electrical resistance changes and the out of balance signal is fed to a suitable amplifier. The output from the amplifier is either digitized, and the binary number sent to a computer for storage, or the output is passed directly to a potentiometric recorder. The detector actually measures the electrical resistance between the electrodes which by suitable non-linear amplification, can be made to provide an output that is linearly related to solute concentration. It is essential that an AC voltage is used across the electrodes to measure the cell impedance to avoid electrode polarization. The frequency of the AC potential across the electrodes is usually around 10 kHz.
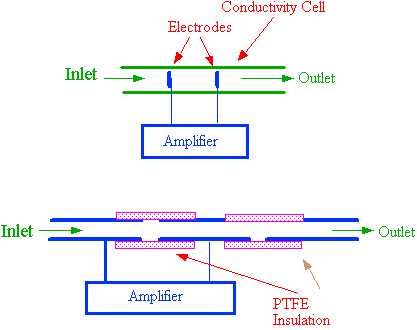
Figure 21. An Electrical Conductivity Detector Sensing Cell
